Children’s Hospital Colorado oncologist Adam Green, MD, understands the risks of whole-brain radiation, but sometimes it’s an important part of the cure for cancers like medulloblastoma or high-risk leukemia. The problem is that in addition to the long-term cognitive challenges that can accompany this use of radiation, the treatment can actually cause new cancers: About 3 percent of patients who receive whole-brain radiation will go on to develop a high-grade glioma 5-12 years after treatment. Unfortunately, these treatment-induced gliomas tend to be more aggressive than the original tumors, and even more aggressive than high-grade gliomas that arise spontaneously.
“It’s great to cure their childhood cancer, but if eight years later they’re getting a fatal brain tumor due to their treatment, that’s something we need to improve on,” Green says. Addressing the problem posed by treatment-induced gliomas is twofold: Develop new strategies to target these cancers and discover what predisposes some patients to grow these tumors so that doctors can prevent them from forming in the first place. Due to the foresight of Children’s Colorado researcher, Nicholas Foreman, MD, Colorado is in a unique position to do both. “Twenty-five years ago, Nick and his team started to collect tumor samples from patients, and now we have one of the largest libraries of these samples in the world,” Green says. “What it allows us to do is to see how these treatment-induced gliomas are different from the ones that arise spontaneously—what genetic changes drive them to grow and how might we better treat them.”
Colorado has the researchers. It has the tumor samples. The sticking point has been the funding that would allow the researchers to work with the tumor samples. “The support of The Morgan Adams Foundation has been essential in helping us do the work that could lead to new strategies against these uniformly fatal cancers,” Green says.
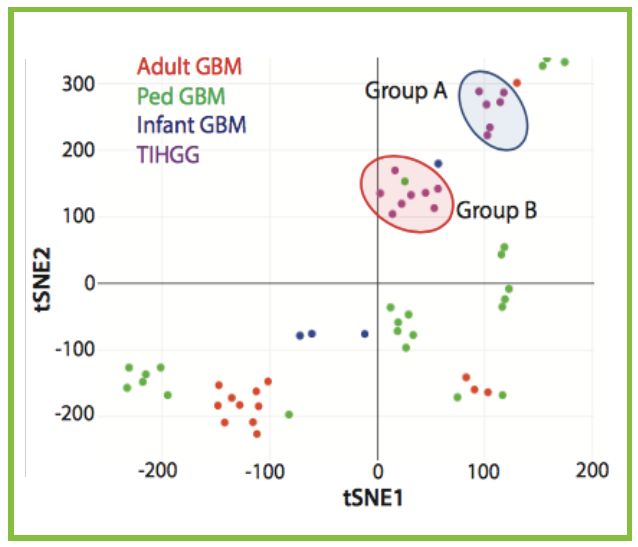
There are many steps along the path to a new treatment against cancer. The first is often an observation: “Like everything I work on, this project comes from a clinical issue,” Green says, namely the observation of high-grade gliomas that occur years after intracranial radiation. The second step is frequently basic science that explores the causes of this observation – Green and his Colorado colleagues have been working with collaborators at St. Jude’s Children’s Research Hospital to discover what makes these cancer unique, finding that, “these tumors are mostly different from spontaneous high-grade gliomas; they have unique genetic changes that drive them to grow,” he says. And Green has even taken the next, next step, “finding promising medicines these tumors may be susceptible to.”
Now Green and his team are moving on to testing these medicines. As you might guess, testing doesn’t start in humans. “Our next step is using patient samples to make patient-derived mouse models to investigate what treatments will be effective. We think the best treatments will likely be different than the treatments we use in spontaneous high-grade gliomas,” Green says. And The Morgan Adams Foundation continues to support this work, helping Green to show that in addition to treatment-induced gliomas being different from gliomas that arise spontaneously, not all treatment-induced gliomas are created equal. In fact, he thinks there are probably two subtypes, one that happens in kids with potential genetic susceptibilities.
“There may be early risk signals, a kind of genetic susceptibility, but there’s a lot more work to do to know for sure,” Green says. “We think there may be subtle genetic risk factors – not something that can cause cancer on its own, but when you’re exposed to radiation therapy, it could be what’s causing these high-grade gliomas.” The second subtype seems more random. Importantly, in the group’s cancer cell experiments, these two subtypes respond differently to chemotherapy.
“My hope for the eventual next step is to have a backbone of standard treatments that will be effective against all of these treatment-induced gliomas, and then combine this backbone with personalized medicine to look at the specific genetic drivers of each tumor, and match that up to a targeted medicine,” Green says.

The Green Lab
From left to right: John DeSisto, Senior PRA, PhD Candidate; Adam Green, MD, Principal Investigator; Hannah Chatwin, PRA; Aaron Knox, PhD, Senior PRA
The Morgan Adams Foundation Pediatric Brain Tumor Research Program
at the University of Colorado Anschutz Medical Campus / Children’s Hospital Colorado

