 Medulloblastoma is a type of malignant brain tumor that is typically diagnosed in children and adolescents. It is one of the most common brain tumors seen in kids and accounts for 15-20% of all pediatric brain tumors.
Medulloblastoma is a type of malignant brain tumor that is typically diagnosed in children and adolescents. It is one of the most common brain tumors seen in kids and accounts for 15-20% of all pediatric brain tumors.
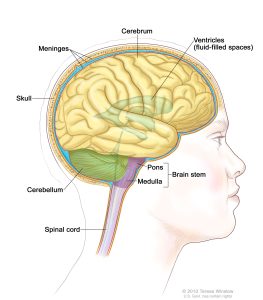
Medulloblastoma tumors usually form in the cerebellum. The cerebellum is the part of the brain that controls balance, coordination, and other complex motor functions.
When a tumor grows into or presses on an area of the brain, it may stop that part of the brain from working the way it should. Symptoms of medulloblastoma often include headaches, nausea, vomiting, clumsiness, and problems with motor skills.
Medulloblastoma is a heterogeneous brain tumor, both clinically and genomically:
- Clinical heterogeneity means there is a wide array of characteristics that are seen in patients, including gender, age of patients at diagnosis, severity of disease, treatment, and outcomes.
- Genomic heterogeneity means there are many different genetic mechanisms that result in medulloblastoma tumor formation. Because of this, there are four distinct subgroups of medulloblastoma and multiple subtypes within the subgroups.
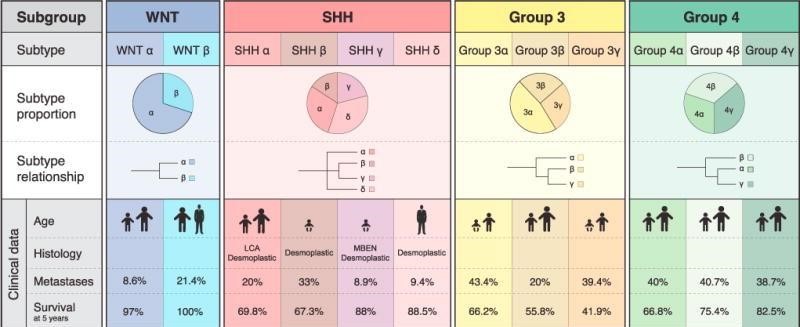
There is a wide variance in outcomes for kids with medulloblastoma. Of the four medulloblastoma subgroups, Group 3 tumors have the worst prognosis and high rates of metastasis (spread to other areas of the brain and spine).
Patients with Group 3 medulloblastoma are more likely to develop recurrent tumors, which are far more aggressive. Kids whose tumors recur have a five-year survival rate around 10%.
Patients with recurrent medulloblastoma have a very poor prognosis. When it returns, the cancer has developed resistance to radiation treatment and no longer responds to standard therapeutic options. Currently, there is very little research investigating tumor recurrence in medulloblastoma.
 Bethany Veo, PhD is working to understand and find ways to target the mechanisms responsible for radiation resistance in Group 3 medulloblastoma.
Bethany Veo, PhD is working to understand and find ways to target the mechanisms responsible for radiation resistance in Group 3 medulloblastoma.
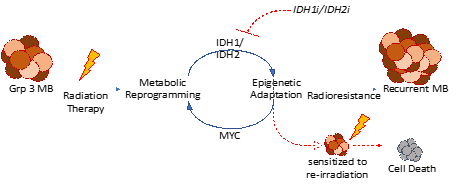
Group 3 medulloblastoma tumors exhibit high levels of a gene called MYC. MYC is an oncogene associated with many different types of cancer. MYC has a role in transcription, which is the process of making an RNA copy of a gene (DNA) sequence.
The overabundance of MYC in medulloblastoma controls several processes that aid in the cancer cells’ ability to grow and resist standard therapeutic treatments such as radiation.
IDH1 is a metabolic pathway that enables Group 3 medulloblastoma tumors to become resistant to radiation. IDH1 has been shown to promote tumor proliferation and alter the way DNA is packaged, which makes the cells radiation-resistant.
Dr. Veo hypothesized that radiation resistance is promoted by metabolic reprogramming made possible by MYC and IDH1, and that inhibiting IDH1 will make medulloblastoma cells susceptible to radiation treatment and prevent further development of resistance.
The goal of this research is to understand how these cells acquire resistance and determine if disruption of the cells’ reprogramming with an IDH1 inhibitor will provide an effective treatment for kids with recurrent medulloblastoma.
Once complete, this work is expected to lead to a clinical trial testing a new combination treatment for kids with recurrent medulloblastoma using IDH inhibitors and radiation.
This research is critical because it has the potential to provide viable treatment options to kids and teens with recurrent medulloblastoma who currently have very little hope.
Because of donor support, kids with recurrent medulloblastoma are no longer being overlooked and new treatments to overcome radiation-resistance are being developed.
This project is laying the groundwork for a better understanding of how this type of medulloblastoma returns and evades treatment. Kids affected by this deadly cancer desperately need our attention and this work is just the beginning of what is possible.
With continued support, this new treatment will hopefully become the next clinical trial for kids with Group 3 medulloblastoma.

