High-grade glioma (HGG) is the leading cause of cancer death among children. HGGs are fast-growing tumors in the brain or spinal cord that arise from glial cells. Glioblastoma (GBM) and diffuse midline glioma (which includes most diffuse intrinsic pontine gliomas or DIPGs) are two types of high-grade glioma most often seen in children. 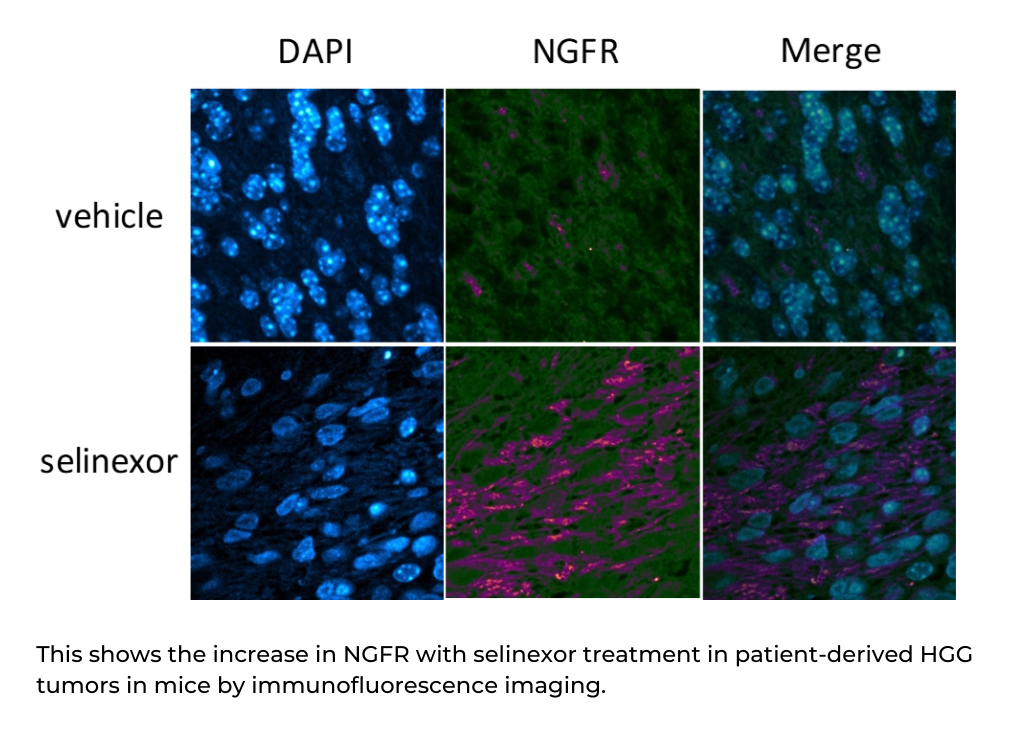
Led by physician-scientist Dr. Adam Green and John DeSisto, a senior professional research assistant (PRA) and PhD student in his lab, a team of researchers in the Morgan Adams Foundation Pediatric Brain Tumor Research Program has been investigating new and more effective treatment options for kids with these very challenging and deadly tumors.
Selinexor, a novel cancer drug, was recently approved by the FDA for an adult cancer called multiple myeloma and is in clinical trials for other cancers, including high-grade glioma. Selinexor inhibits a protein that exports tumor suppressor proteins (the brakes in cells) from the nucleus where they function so that tumor cells can grow without restraint. Dr. Green and his team studied patient-derived cell lines and mouse models to understand how selinexor works in HGG and found that selinexor decreases tumor proliferation and increases cancer cell death.
The process to achieve HGG tumor cell death involves a few steps: selinexor increases the expression of a protein called NGFR, which then blocks the activation of a pathway called NF-kB that regulates cell proliferation and survival and is found in many types of cancer. This mechanism of action suggests potential combination therapies that may work cooperatively with selinexor against high-grade glioma.
In the mouse model studies conducted, the cancer cells eventually built up a resistance when treated with selinexor alone, so researchers screened selinexor with a panel of FDA-approved anti-cancer agents. Bortezomib is a proteasome inhibitor that inhibits the NF-kB pathway through a different mechanism than selinexor, and when used in conjunction, the two drugs worked synergistically against HGG in lab studies.
The study, recently published in Molecular Cancer Therapeutics, is important in the effort to find effective treatments for kids with a high-grade glioma. Dr. Green is currently leading a national phase 1 clinical trial of selinexor for children with recurrent cancers, with a specific focus on HGG. This work provides valuable insight into how to confirm selinexor is working in human tumors, and suggests a method for combining other drugs to make selinexor even more effective in future trials. “The potential for combination treatment with medicines that are already being used in patients and are easily translatable to clinical trials for these currently incurable tumors is very exciting,” says Green.
This work is the collaborative effort of members of the Green, Venkataraman, and Vibhakar Labs in the Morgan Adams Foundation Pediatric Brain Tumor Research Program at the University of Colorado Anschutz Medical Campus / Children’s Hospital Colorado, in addition to assistance from investigators at Vanderbilt University, Memorial Sloan Kettering Cancer Center, and Karyopharm Therapeutics (maker of selinexor). The future steps for this project include studying the combination of selinexor and proteasome inhibition in patient-derived mouse models and validating the markers of efficacy in mouse and human tumor samples.
More information: John A. DeSisto et al., “Exportin 1 inhibition induces nerve growth factor receptor expression to inhibit the NF-kB pathway in preclinical models of pediatric high-grade glioma,” Mol Cancer Ther (2019).
https://mct.aacrjournals.org/content/early/2019/10/08/1535-7163.MCT-18-1319
Journal Information: Molecular Cancer Therapeutics

The Green Lab
From left to right: John DeSisto, Senior PRA, PhD Candidate; Adam Green, MD, Principal Investigator; Hannah Chatwin, PRA; Aaron Knox, PhD, Senior PRA
The Morgan Adams Foundation Pediatric Brain Tumor Research Program
at the University of Colorado Anschutz Medical Campus / Children’s Hospital Colorado

