As technology continues to advance and researchers learn more about how specific types of cancer form, grow, and respond to treatment, an increasing number of specific subtypes of cancer are being identified.
The primary advancement in classifying all types of cancer currently comes from molecular profiling. Pediatric cancer is no exception and many new brain tumor subtypes affecting kids have been discovered and re-classified through a better understanding of the genome.
 David Solomon, MD, PhD is a physician-scientist and Assistant Professor in the Department of Pathology at the University of California San Francisco. Dr. Solomon specializes in molecular diagnostics for brain tumors and directs a research laboratory focused on deciphering the molecular pathogenesis and advancing precision medicine treatment of childhood brain tumors and sarcomas.
David Solomon, MD, PhD is a physician-scientist and Assistant Professor in the Department of Pathology at the University of California San Francisco. Dr. Solomon specializes in molecular diagnostics for brain tumors and directs a research laboratory focused on deciphering the molecular pathogenesis and advancing precision medicine treatment of childhood brain tumors and sarcomas.
His laboratory is composed of a talented group of cell biologists, computational data scientists, and clinical research fellows who employ a combination of cutting-edge genomics and epigenomics technologies, cell culture models, and genetically engineered mouse strains to investigate pediatric brain tumors and other childhood cancers.
In just the last 36 months, many new subtypes of pediatric tumors have been discovered, including several because of your support of Dr. Solomon’s groundbreaking research:
- Sarcomas and brain tumors driven by rearrangements in a gene called BCOR
- CNS embryonal tumors with PLAGL1/2 gene amplification
- Intracranial mesenchymal tumors with FET-CREB fusions
- A new pediatric subtype of primary central nervous system lymphoma
- A new distinct subtype of astroblastoma, a type of glioma brain tumor
In addition to the above new classifications of pediatric cancers, Dr. Solomon and his team have also made significant discoveries from their study of gliomas that arise in the setting of neurofibromatosis type 1 (NF1).
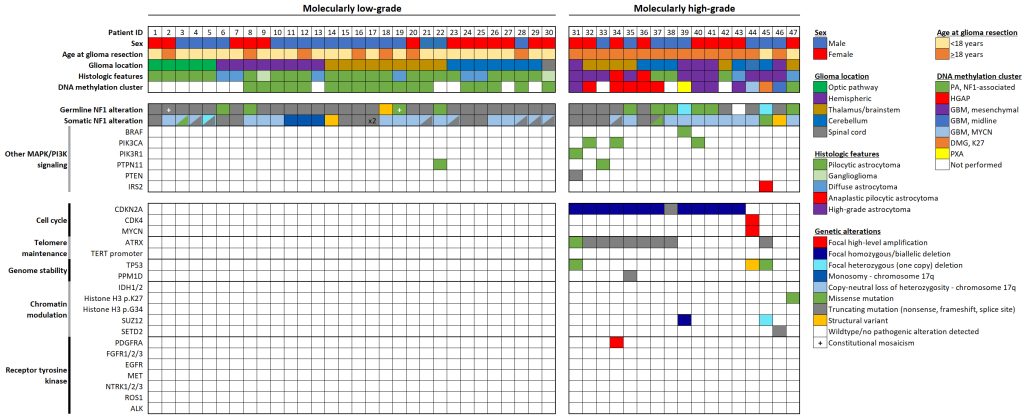
From this work, they have shown that gliomas in kids with NF1 are unique and distinct from those gliomas that arise spontaneously. Because of their differences from spontaneous glioma brain tumors, patients with NF1-associated gliomas likely benefit from different treatments.
Additionally, Dr. Solomon’s team identified two distinct molecular subgroups of gliomas in NF1 patients, each with different clinical signs and outcomes. One subgroup of NF1-associated gliomas occurs primarily during childhood, are molecularly low-grade, and appear to benefit from MEK inhibitors. The second subgroup occurs primarily during adolescence and early adulthood, are more aggressive clinically, are molecularly high-grade, and require new combinatorial treatment approaches.
We now increasingly recognize that a substantial number of brain tumors in children are not spontaneous, but instead occur as part of different inherited genetic tumor syndromes, such as Lynch syndrome, Li-Fraumeni syndrome, neurofibromatosis type 1 and 2, and several others.
It is only through dedicated genomic testing of each individual child with cancer that we are able to assess the contribution of any inherited genetic variants that might be causing increased cancer risk and potentially alter clinical management.
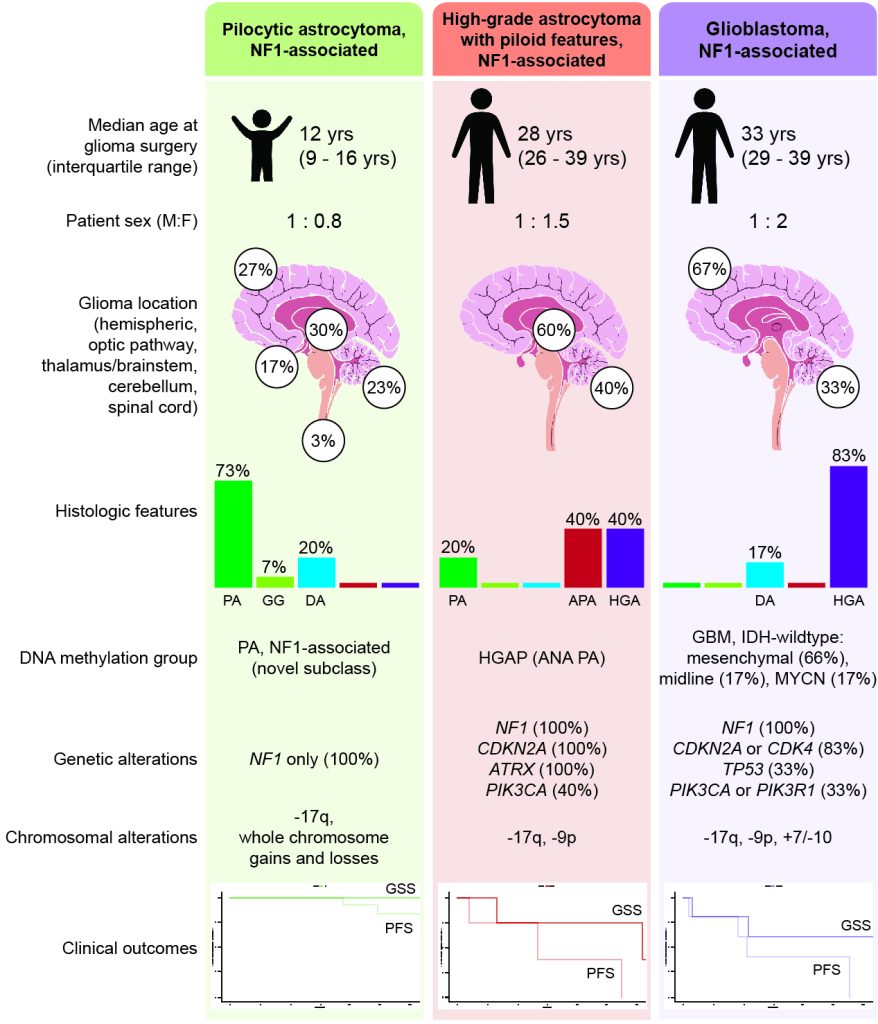
Advancements like what are happening with Dr. Solomon’s research team have contributed to more precise methods of classifying and treating pediatric brain tumors. The combination of conventional histopathology (studying tumors under a microscope) with cutting-edge genomic profiling has become the standard of care for diagnosing neuro-oncology patients in the last few years.
These efforts to accurately diagnose patients are the basis for “precision medicine” and providing treatment based on the most precise information available. Identifying tumor subtypes is not merely a matter of naming the type of cancer – it is hugely important for creating clinical trials that are focused on improving outcomes for kids based on their specific tumor type. Treatments that work for one tumor type may not be effective in another, and that’s why this work is so important.
For kids with rare tumor subtypes, research is the only way forward to find treatments that will work and give them a chance at survival.
Donor support is enabling Dr. Solomon and his research team to continue working toward identifying and understanding the different types of pediatric cancers so that kids can be treated and get to the cancer-free future they deserve.
The Solomon Lab
University of California San Francisco


