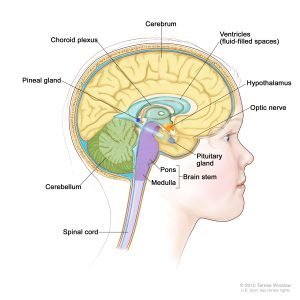
Craniopharyngioma is a type of pediatric brain tumor that typically affects kids under the age of 14 (although it also affects adults between 40-75 years). This tumor is unique in that it usually contains both solid and cyst-like components. Craniopharyngioma most often occurs at the base of the brain behind the eyes near the pituitary gland.
Craniopharyngiomas are benign tumors, which means they do not spread to other locations in the body the way malignant, cancerous tumors do. Although they do not spread, craniopharyngiomas growing in the brain cause serious problems.
The standard treatment for these tumors is surgical resection (complete or partial) with or without radiation therapy. Kids with craniopharyngioma often experience a wide range of devastating symptoms including vision loss, cognitive impairment, obesity, delayed puberty, abnormally slow growth, anxiety, and much more. Craniopharyngioma patients have some of the poorest quality of life among kids with brain tumors.
Dr. Todd Hankinson is a pediatric neurosurgeon and researcher who has spent the last decade advancing treatment and care of kids and teens with brain tumors. Dr. Hankinson created and leads the only multi-center consortium in North America that focuses solely on improving therapies for pediatric craniopharyngioma.
the last decade advancing treatment and care of kids and teens with brain tumors. Dr. Hankinson created and leads the only multi-center consortium in North America that focuses solely on improving therapies for pediatric craniopharyngioma.
Your support of The Morgan Adams Foundation has helped enable Dr. Hankinson’s research since 2013. Specifically, Dr. Hankinson is searching for more therapies that can be offered to craniopharyngioma patients outside of radiation to improve their survival and quality of life.
Dr. Hankinson and his team’s research has led to a new clinical trial of a combination treatment for kids with craniopharyngioma. Here’s what they are working on:
Analysis of craniopharyngioma tumor tissue and cyst fluid indicated high levels of interleukin-6 (or IL-6) and IL-6 receptors. IL-6 is a type of protein that regulates the immune response in the body.
Tocilizumab is a drug that blocks the IL-6 protein and has been widely used in pediatric patients. Dr. Hankinson treated two craniopharyngioma patients at Children’s Hospital Colorado with tocilizumab under the FDA’s compassionate use guidelines and both responded well and experienced decreased tumor size.
Research collaboration leads to combination therapy clinical trial
Dr. Hankinson’s team collaborates with other teams researching craniopharyngioma around the world, contributing data and sharing ideas. One such collaboration is with Dr. Juan Pedro Martinez-Barbera at University College London. (In fact, MAF funded two joint projects between Drs. Hankinson and Martinez-Barbera in 2016!)
Dr. Martinez-Barbera’s research team in London recently demonstrated that a type of drug called MEK inhibitors may slow craniopharyngioma growth.
Building off both teams’ discoveries, Dr. Hankinson created a clinical trial of IL-6 inhibition using tocilizumab that is currently underway at Children’s Hospital Colorado.
He is also working with a pediatric neuro-oncology consortium called CONNECT who will soon open an international clinical trial for kids with craniopharyngioma.
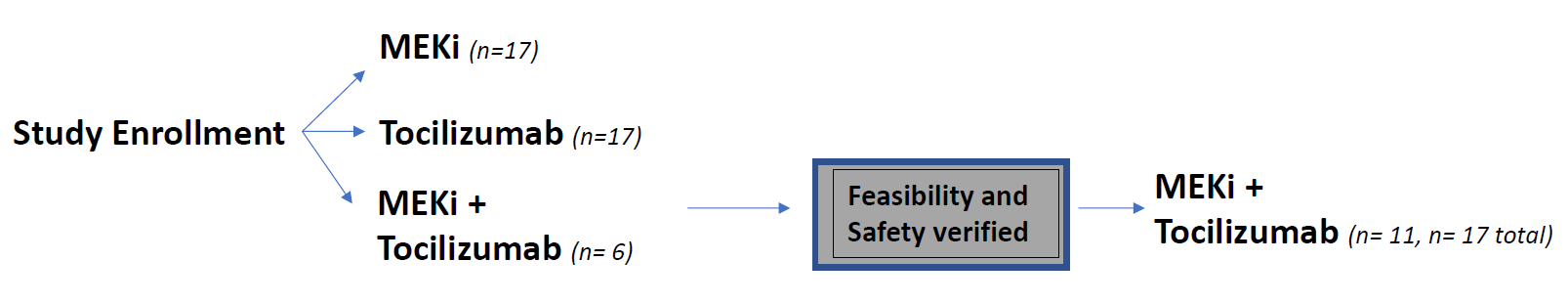
This trial will combine tocilizumab treatment with a MEK inhibitor. The trial is in the approval phases and will begin enrolling patients soon.
There will be three groups of patients in the trial. One will receive tocilizumab treatment, another will receive the MEK inhibitor, and the final group will receive a combination of both tocilizumab and the MEK inhibitor.
This new clinical trial will hopefully be the first of several that become available for craniopharyngioma patients who for so long have had very few treatment options. Surgery combined with radiation treatment is currently the primary option available and often results in a number of debilitating side effects on children’s brains.
In addition, this trial will lay the groundwork for future clinical trials utilizing currently-available drugs that may help improve outcomes for kids with this very aggressive brain tumor.