Generally, there are two ways to discover a new cancer drug: You can use basic science to discover a cancer’s Achille’s heel and then find or build a drug that exploits it, or you can try a bunch of drugs on cancer cells and see what works. Think of the basic science approach as building from the bottom-up and testing many drugs as working from the top-down. Your support of The Morgan Adams Foundation is enabling researchers at Children’s Hospital Colorado to do both.
And, unfortunately, both approaches are needed. The cancer called atypical teratoid/rhabdoid tumor (AT/RT) is a rare brain cancer primarily affecting children under three, with a 5-year survival rate below 30 percent. A group in the Morgan Adams Foundation Pediatric Brain Tumor Research Program has been working toward new AT/RT treatments from both directions.
The team, led by Jean Mulcahy-Levy, MD and funded by The Morgan Adams Foundation, started by gathering patient samples of AT/RT and treating these cell samples with 134 FDA-approved anti-cancer drugs.
“Our goal was to identify already-FDA approved drugs that could be rapidly moved from preclinical data to a clinical trial,” Mulcahy-Levy says.
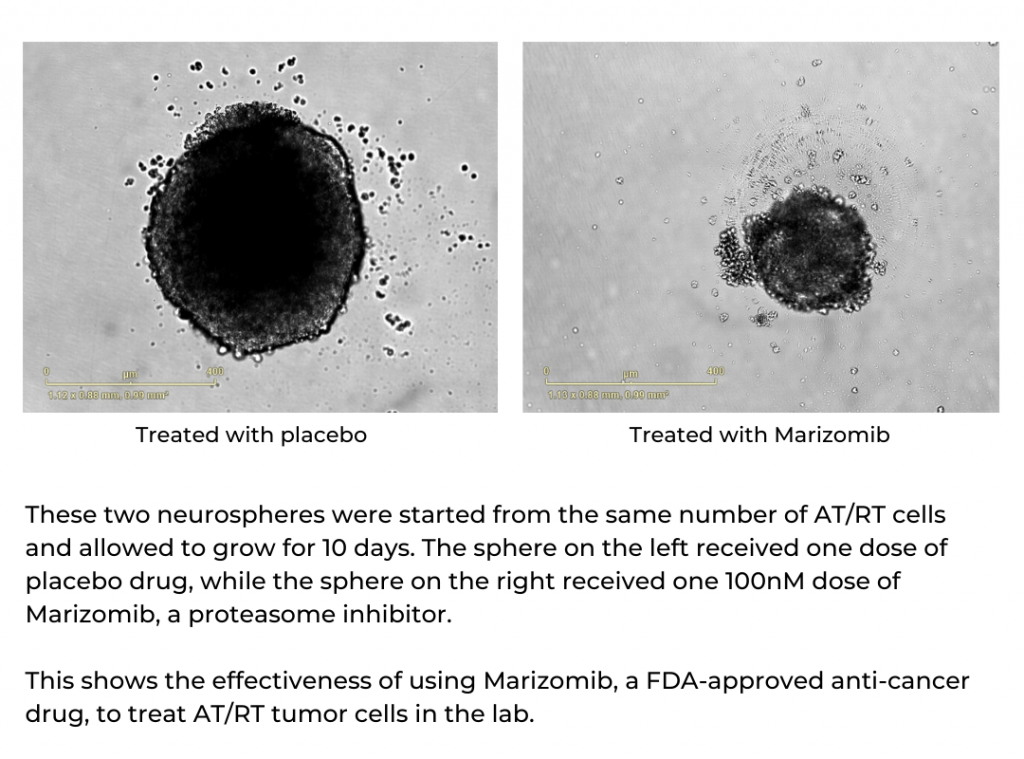
The drug that most efficiently killed AT/RT cells is called marizomib, one in a relatively new class of drugs called proteasome inhibitors. Importantly, marizomib can pass through the blood-brain barrier, meaning that in addition to treating AT/RT cells in a dish, it is much more likely to reach the cancer cells in a patient’s brain.
“The most important thing you want to show in the lab is not just that a drug is effective, but that it can actually reach the target in doses that could help patients. After working with cells, we showed marizomib could reach AT/RT brain tumors in mouse models, and we showed that it increased the survival time of these mice,” Mulcahy-Levy says.
Again, successful drug development depends not only on being able to kill cells and save mice, but on the strategy being practical for human patients.
“There are adult trials showing marizomib could potentially work in adult brain tumors, and earlier proteasome inhibitors have been used to treat pediatric leukemia patients. If you bring those two together, there’s the potential of really having a lot of great and quick impact in a population with a really poor survival,” Mulcahy-Levy says.
The group is now writing a clinical trial to test marizomib along with standard-of-care therapy in young AT/RT patients.
“The fact that the NIH will give this drug panel to investigators for free is such an amazing resource. It allows you to test all these drugs one at a time – there are actually 147 drugs now – and by doing these larger screens of currently available drugs, you can shorten the time it takes to bring promising new drugs into clinical trials,” Mulcahy-Levy says.
At the same time, the group is using basic science to understand what makes AT/RT tick, with the goal of learning to turn off the things that cause the disease. As often happens, the hunt began with the discovery of something that is different in many AT/RT cells than in healthy cells. Specifically, AT/RTs lose function in a gene called SMARCB1.
But that presents a problem: Doctors use drugs to turn genes off, but there’s no direct way to restore function in a gene silenced by cancer. So, the team looked a level deeper. And they found an important collaborator: The gene CDK7 may work with loss of SMARCB1 to supercharge AT/RT tumors. And while there are no FDA-approved drugs targeting CDK7 yet, there are a handful in drug-development pipelines.
“SMARCB1 mutation is seen in almost every single one of these tumors and we think CDK7 inhibitors will specifically interact with this mutation,” Mulcahy-Levy says.
While the basic science work was delayed by COVID19, “we have 3-4 months of research that we need to apply for an NIH grant that could lay the groundwork for a clinical trial of this strategy, too,” Mulcahy-Levy says.
It may even be possible to combine the top-down discovery of marizomib with the bottom-up discovery of CDK7 inhibition.
“We take an older drug, combine it with a newer drug, and then work toward combining those together. It’s possible we could combine these two therapies to make these treatments even better,” Mulcahy-Levy says.
While the drug-development strategies may be interesting, it is the results that are essential. Whether marizomib, a CDK7 inhibitor, or both, this work holds great promise to provide new treatment options for young patients who desperately need them.
The Morgan Adams Foundation Pediatric Brain Tumor Research Program
at the University of Colorado Anschutz Medical Campus / Children’s Hospital Colorado

The Mulcahy-Levy Lab
From left to right: Michele Desmarais, BS, Professional Research Assistant; Andrew Morin, MS, Senior Professional Research Assistant; Shadi Zahedi, MS, Senior Professional Research Assistant;

Jean Mulcahy-Levy, MD, Principal Investigator

